Geology Geek Time!
-
RE: https://blackqueer.life/@FinalGirl/116061945653296406
Okay, now that we started with learning how to start with a ternary diagram, we're going to talk about… GASP! … Chemistry!
But not really. Don't worry. This is, like, cereal box chemistry.
Okay, so recall from my epically long post about pumice, geologists have a best friend element called silicon. Chemical symbol "Si".
This happens to bond often with Oxygen (O) to form Silicon Dioxide. Di is "two." Dioxide is "Two oxygens." Silicon Dioxide is SiO2, a molecule made of one silicon atom, two oxygen atoms. We're lazy so we just call this silica. We also call it "Quartz." Quarts is just lots of silicon molecules put together in a latice pattern, it's called a mineral.
Now, it turns out that in the presence of other elements, silicon and oxygen will bind to some of them. But… there's a bit of a catch.
Silicon can bind with two oxygen because they reach a state where they don't need to have anything else (cereal box chemistry folks, let it go). But with other elements, that ratio of 1:2 will be difference, because atoms fit together differently.
So let's add some aluminum (Al). If we add aluminum to a soup of silicon and oxygen, we might get Kyanite, Al2SiO5. Kyanite is an aluminum silicate. Now we have one silicon and five oxygens, because we have two aluminums, so it balances with a different silicate ratio.
But… what's important is that it's still a silicate! Silicon and oxygen bonded together is the "base." Add other types of elements to that base and you get other minerals.
And it turns out, there are a bunch of aluminum silicates! Some of those are…
…wait for it…
Feldspars!
Okay, now we understand ternary diagrams, and we understand aluminum silicate chemistry. So we can read this ternary digram of alumium silicate chemistry, right?
Okay, maybe not quite, but lets talk through it.
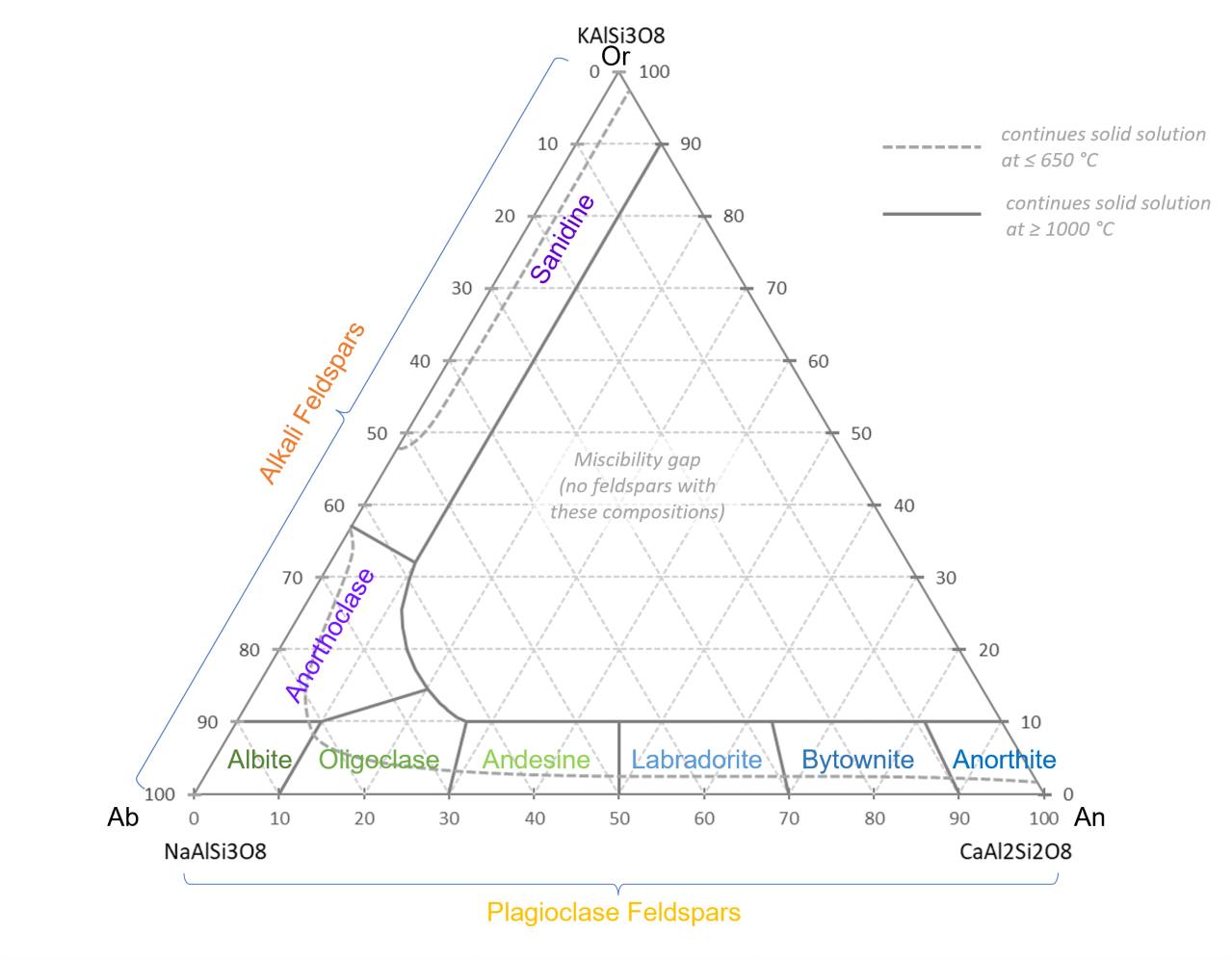
In our ternary diagram, we have three end-members: Potassium (K), sodium (Na), and calcium (Ca).
Recall from our ternary diagram introduction, if we put a point anywhere on the diagram, we will have a plot of concentrations of those three elements.
It turns out that if we go all the way to the bottom right corner, we have Calcium aluminum silicate (Anorthite), then the Ca atom adds a different "balance" to the molecule, so we have CaAl2Si208. One calcium atom, two aluminum atoms, two silicon atoms, and eight oxygen atoms.
So, at 100% calcium, that's what things look like. But as we go along the bottom of the chart from right to left, we have a little bit more sodium and, and a little more sodium. For instance, if you are on the 90% point across the bottom (90% calcium), you can follow the dotted line from that point up to the left side of the graph to see that's also 10% sodium. And as you go down in calcium, you go up in sodium.
If we go all the way to bottom left, with 100% sodium, we have a different chemical formula: NaAlSi308. One sodium, one aluminum, three silicon, eight oxygen.
Each of these collections of a bit of calcium and a bit of sodium is a different mineral. 50-70% calcium and you have labradorite (a very beautiful feldspar with a lovely iridescent sheen).
And again, you can go from sodium to potassium. Increasing in potassium and decreasing in sodium gives you different minerals until you get to all potassium aluminum silicate, KAlSi3O8.
Those are all the feldspars! Now, you not only know their names, you know they are all aluminum silicates, and all have different percentages of calcium, sodium, and potassium.
-
Okay, now we understand ternary diagrams, and we understand aluminum silicate chemistry. So we can read this ternary digram of alumium silicate chemistry, right?
Okay, maybe not quite, but lets talk through it.
In our ternary diagram, we have three end-members: Potassium (K), sodium (Na), and calcium (Ca).
Recall from our ternary diagram introduction, if we put a point anywhere on the diagram, we will have a plot of concentrations of those three elements.
It turns out that if we go all the way to the bottom right corner, we have Calcium aluminum silicate (Anorthite), then the Ca atom adds a different "balance" to the molecule, so we have CaAl2Si208. One calcium atom, two aluminum atoms, two silicon atoms, and eight oxygen atoms.
So, at 100% calcium, that's what things look like. But as we go along the bottom of the chart from right to left, we have a little bit more sodium and, and a little more sodium. For instance, if you are on the 90% point across the bottom (90% calcium), you can follow the dotted line from that point up to the left side of the graph to see that's also 10% sodium. And as you go down in calcium, you go up in sodium.
If we go all the way to bottom left, with 100% sodium, we have a different chemical formula: NaAlSi308. One sodium, one aluminum, three silicon, eight oxygen.
Each of these collections of a bit of calcium and a bit of sodium is a different mineral. 50-70% calcium and you have labradorite (a very beautiful feldspar with a lovely iridescent sheen).
And again, you can go from sodium to potassium. Increasing in potassium and decreasing in sodium gives you different minerals until you get to all potassium aluminum silicate, KAlSi3O8.
Those are all the feldspars! Now, you not only know their names, you know they are all aluminum silicates, and all have different percentages of calcium, sodium, and potassium.

@FinalGirl thank you, i love this
-
Okay, now we understand ternary diagrams, and we understand aluminum silicate chemistry. So we can read this ternary digram of alumium silicate chemistry, right?
Okay, maybe not quite, but lets talk through it.
In our ternary diagram, we have three end-members: Potassium (K), sodium (Na), and calcium (Ca).
Recall from our ternary diagram introduction, if we put a point anywhere on the diagram, we will have a plot of concentrations of those three elements.
It turns out that if we go all the way to the bottom right corner, we have Calcium aluminum silicate (Anorthite), then the Ca atom adds a different "balance" to the molecule, so we have CaAl2Si208. One calcium atom, two aluminum atoms, two silicon atoms, and eight oxygen atoms.
So, at 100% calcium, that's what things look like. But as we go along the bottom of the chart from right to left, we have a little bit more sodium and, and a little more sodium. For instance, if you are on the 90% point across the bottom (90% calcium), you can follow the dotted line from that point up to the left side of the graph to see that's also 10% sodium. And as you go down in calcium, you go up in sodium.
If we go all the way to bottom left, with 100% sodium, we have a different chemical formula: NaAlSi308. One sodium, one aluminum, three silicon, eight oxygen.
Each of these collections of a bit of calcium and a bit of sodium is a different mineral. 50-70% calcium and you have labradorite (a very beautiful feldspar with a lovely iridescent sheen).
And again, you can go from sodium to potassium. Increasing in potassium and decreasing in sodium gives you different minerals until you get to all potassium aluminum silicate, KAlSi3O8.
Those are all the feldspars! Now, you not only know their names, you know they are all aluminum silicates, and all have different percentages of calcium, sodium, and potassium.

Now, if you remember my other thread, you can see the "Miscibility gap."
Recall that miscible is just fancy word for "mixable."
Sugar and water are miscible, they mix.
Oil and water are immiscible, they do NOT mix
This is similar to potassium and calcium in an aluminum silicate. It turns out that you will never get a feldspar with, say, 30% calcium and 40% potassium. It doesn't happen. As soon as you have more than about 10% of one of those, you just won't really get any of the other.
Now, if you're astute, you'll notice the anorthoclase/oligoclase boundary. At about 65% sodium, there's the ability to have just a touch more mixing than 10%, but not much.
-
Now, if you remember my other thread, you can see the "Miscibility gap."
Recall that miscible is just fancy word for "mixable."
Sugar and water are miscible, they mix.
Oil and water are immiscible, they do NOT mix
This is similar to potassium and calcium in an aluminum silicate. It turns out that you will never get a feldspar with, say, 30% calcium and 40% potassium. It doesn't happen. As soon as you have more than about 10% of one of those, you just won't really get any of the other.
Now, if you're astute, you'll notice the anorthoclase/oligoclase boundary. At about 65% sodium, there's the ability to have just a touch more mixing than 10%, but not much.
Tune in next time, when we stay in the XKCD comic Average Familiarity and talk about Olivine.
Olivine is a magnesium iron silicate! I bet you have a bit of an idea what that means now!
-
Tune in next time, when we stay in the XKCD comic Average Familiarity and talk about Olivine.
Olivine is a magnesium iron silicate! I bet you have a bit of an idea what that means now!
@FinalGirl ooh, i do wanna read about olivine

-
Tune in next time, when we stay in the XKCD comic Average Familiarity and talk about Olivine.
Olivine is a magnesium iron silicate! I bet you have a bit of an idea what that means now!
While Feldspar has three end-members, olivine only has two: Magnesium and iron.
All magnesium is called Forsterite (Mg2Si04) and all Iron is called Feyalite (Fe2Si04).
Now, if we look again at Bowen's reaction series (linked), we see that Olivine fractionates (turns from liquid to solid: crystalizes) at really high temperatures, the same temperatures as calcium rich felspar crystalizes)
So… here's the thing. If you have a rock, a solid rock, and it has a high concentration of olivine and anorthite (calcium-rich feldspar), you know something about the starting composition of the magma from which that rock formed. And that would be different than a rock formed with only muscovite, quartz, and potassium feldspar.
Here's a cool thing: Oceanic crust (the stuff under the oceans formed in The Rift where the Kaiju come from) is very mafic, high in magnesium, iron, things like that. You find olivine in igneous rocks made from oceanic crust. Continental crust (the stuff we live on), has a much lower percentage of those minerals and is highly dominated by quartz.
So… if you have an igneous rock with olivine and calcium feldspar, you can be reasonably sure that igneous rock was made from mantle magma or melting oceanic crust.
https://en.wikipedia.org/wiki/Bowen%27s_reaction_series#/media/File:Bowen's_Reaction_Series.png
-
Geology Geek Time!
There are a bunch of rewrites of the XKCD comic Average Familiarity in my feed today, with everything from transportation engineering to x86 instruction sets. But the original is a classic for a reason:
Because Geology Rocks!
So, in my attempt to be way more geeky about geology, I'm going to post about feldspars, so that you will not only know one or two feldspars, but ALL THE FELDSPARS!! MWAHAHAHAHAHA!!!
@FinalGirl when the TA in my geology class referred to the feldspar specimen as “Kspar” i knew it was a deep rabbit hole
-
@FinalGirl when the TA in my geology class referred to the feldspar specimen as “Kspar” i knew it was a deep rabbit hole
@aizuchi I’m not a fan of Kspar. Why modes Sanidine get a cool nickname but not poor anorthosite?!

-
@aizuchi I’m not a fan of Kspar. Why modes Sanidine get a cool nickname but not poor anorthosite?!

@FinalGirl 🫂 I’m willing to put all the blame on that grad student
-
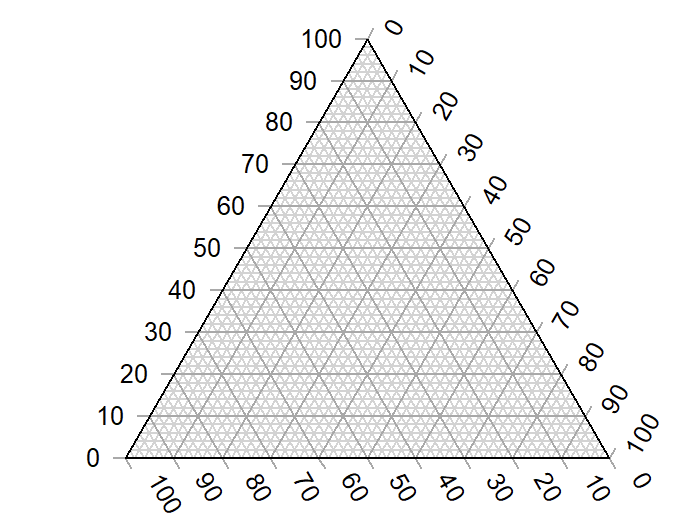
First, we start with our ternary diagram.
Okay, if you're not used to looking at ternary diagrams, we'll start by describing how to start with our ternary diagram.
Let's make an RGB ternary diagram to describe red, green, and blue.
I'm going to teach you first how to create a ternary diagram. "Ternary" is just a geeky name for "three-thinged."
Take a triangle and put the word "Red" at the top. Now draw 9 horizontal lines equally spaced going up it. Now you have "levels." The bottom is zero, first level is 10%, the second level is 20%, and so on.
Now, anywhere you put a dot inside that circle, you have a "percentage of red." Near the top, it might be 85% red. Near the bottom it might be 15% red.
Now, turn the triangle so that a different side is on the bottom. Draw the lines, put "Green" at the top. And again with the last side, draw the lines, and draw "Blue" at the top.
What you end up with is a triangle full of triangles. But what you "really" get is a way to plot a three-coordinate system.
If you put a circle directly in the center, you have 50% on the green scale, 50% on the blue scale, and 50% on the red scale.
Anywhere on the diagram, you have a percentage of all three of those components. That is a ternary diagram.
@FinalGirl this runs the gamut from geology to color theory...
-
R relay@relay.infosec.exchange shared this topic

